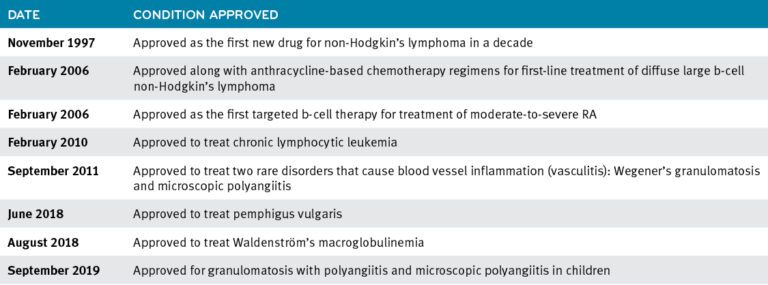
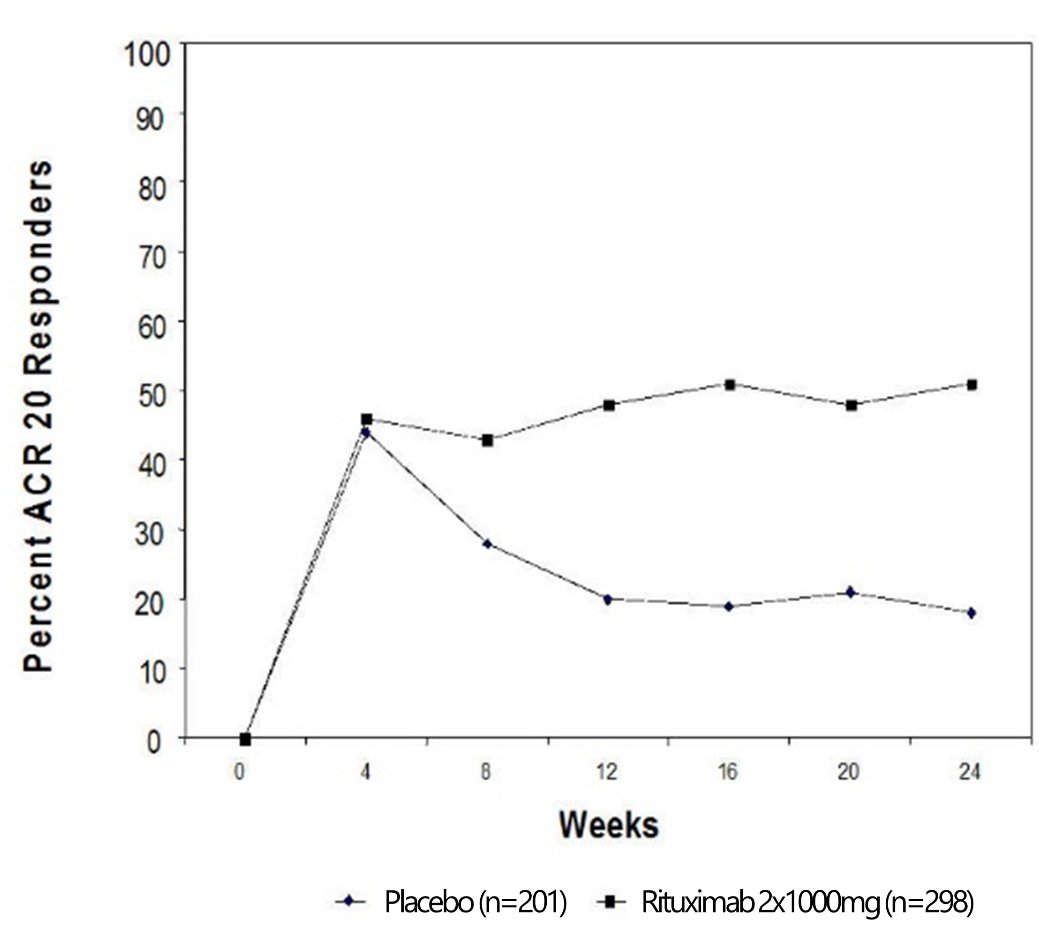
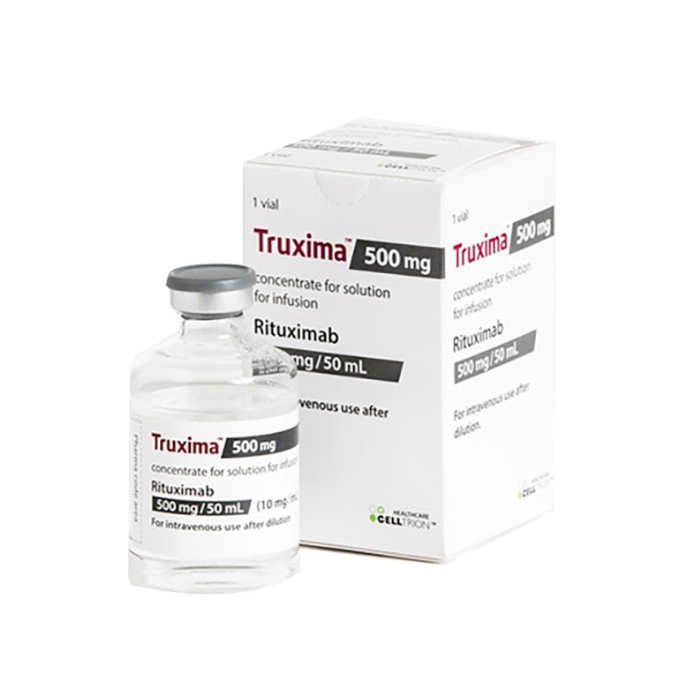
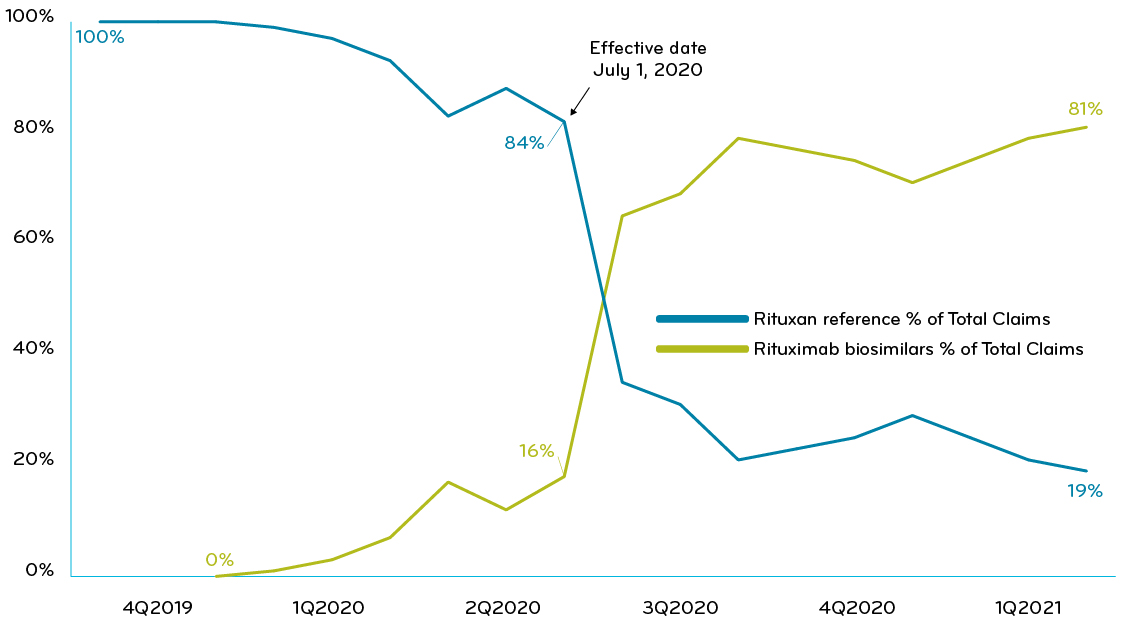
Clinical Oncology News Current Practice FEBRUARY 16, 2022 The Case for Switching to Rituximab Biosimilar Switching from originator rituximab ( Rituxan, Genentech) to rituximab-abbs (Truxima, Teva) can save an institution up to $13,000 annually per ...

Say hello to Roche's worst-case scenario: Teva's Rituxan biosim set to launch in U.S. | Fierce Pharma