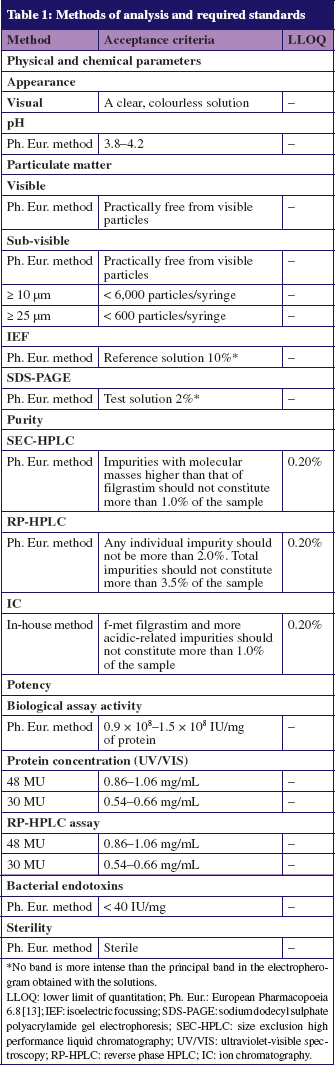
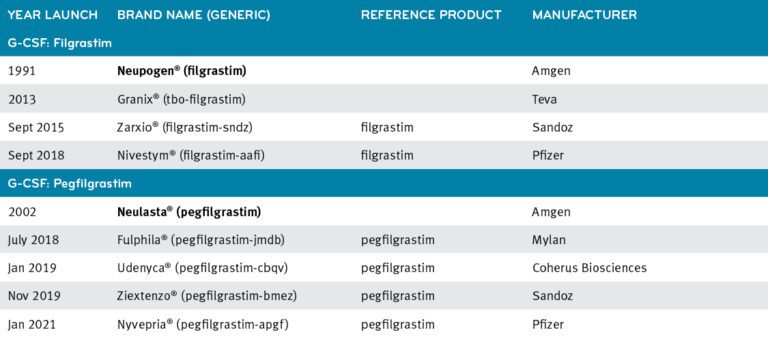
Tevagrastim - Filgrastim 300mcg/0,5ml - Solução Injetável - Teva - ÁgilMed - Medicamentos Especiais e Nutrição Clínica
Key concepts and critical issues on epoetin and filgrastim biosimilars. A position paper from the Italian Society of Hematology, Italian Society of Experimental Hematology, and Italian Group for Bone Marrow Transplantation